Supercapacitors are used as DC energy storage media, short high-power charge storage (automotive start-stop systems), backup for semiconductor memories and microprocessors, etc. New designs in larger modules have opened up space for several power applications that concur with rechargeable batteries.
There is no fixed dielectric material, and the charge is accumulated in the interface between active electrodes and the electrolyte. There are two basic mechanisms of charge storage:
- Electrostatic Charge Storage
- Pseudocapacitance Electrochemical Charge Storage
Electrostatic storage is based on the charge accumulation in charge traps within so-called Helmholtz layers (inner and outer) – see Figure 1. Pseudocapacitance storage is an electrochemical process where adsorbed ions accumulate charge. – see Figure 2.
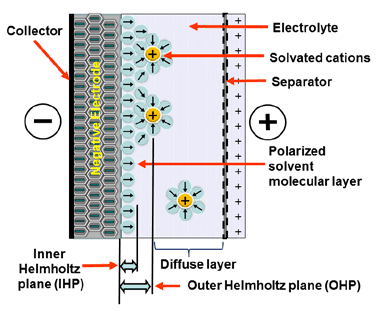
Figure 1. Electrostatic storage charge mechanism of supercapacitors
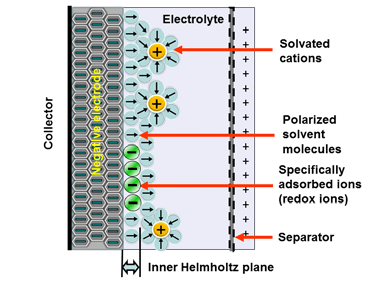
Figure 2. Electrochemical storage (Pseudocapacitance) charge mechanism in supercapacitors
The mix of both charge storage mechanisms is present in real supercapacitors. The dominant charge mechanism that is present in supercapacitors defines three categories as shown in Figure 3.
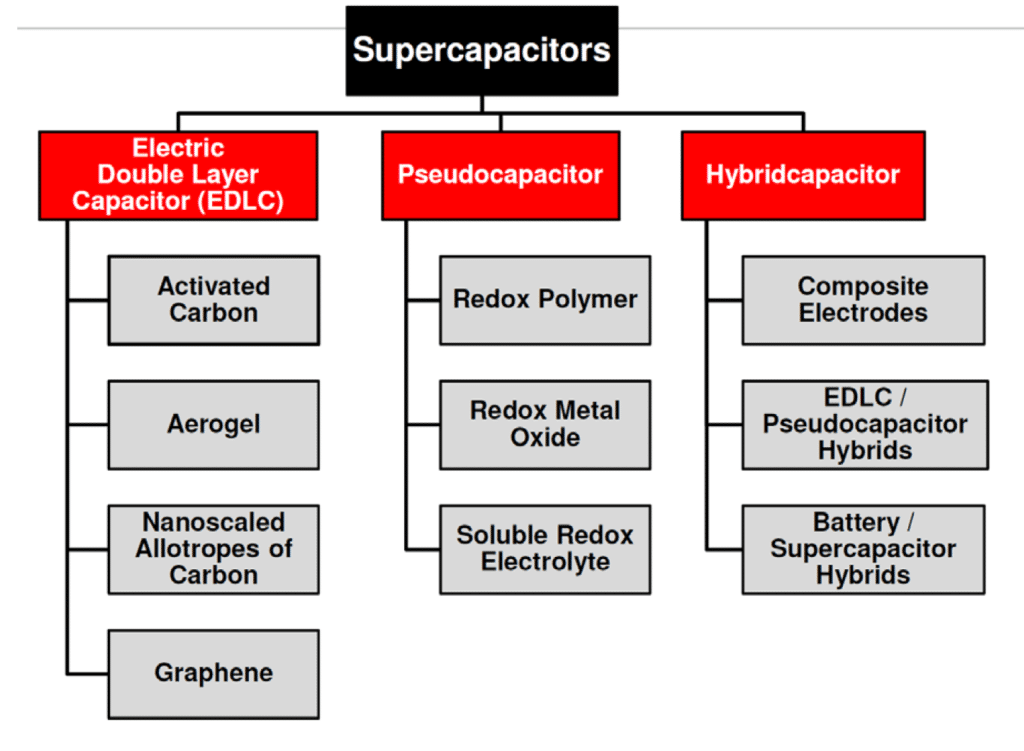
Figure 3. Supercapacitor types
EDLC capacitors use high-surface synthesized electrodes based on activated carbon, carbon nano-tubes, or graphene. Alternatively, the electrodes can be made from cheap “bio-waste” monolithic material with a natural hierarchy of pore sizes, such as coconuts, melon rinds, wood, fish scales, etc.
EDLC capacitors with symmetrical electrodes are non-polarized but are, in practice, supplied with a polarity marking that should be followed. One reason is that the positive electrode (+) may be processed differently from the negative one (-).
The electrochemical storage – pseudocapacitance – is not related to any electrochemical reaction – in difference to batteries. The charge can be stored by mechanisms such as redox-pseudocapacitance or redox-intercalation – see Figure 4. below.
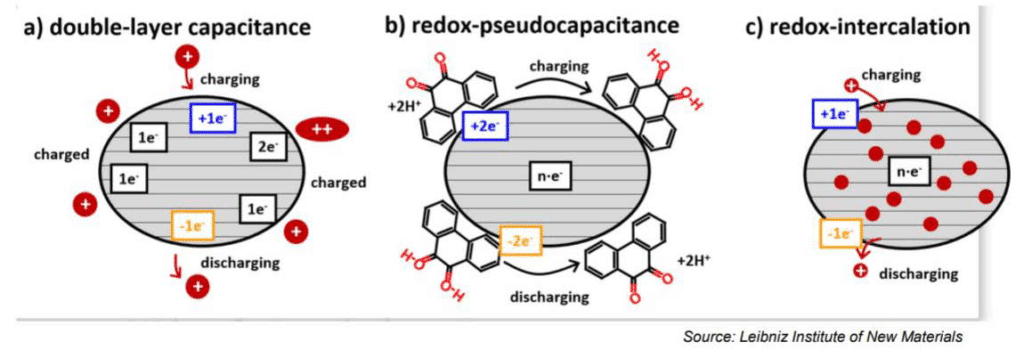
Figure 4. Supercapacitor storage mechanisms
It is also possible to combine hybrid designs with other electrode technology, such as
- capacitor hybrid: wet tantalum hybrid capacitor – one electrode tantalum anode and second electrode supercapacitors
- battery hybrid: supercapacitor one electrode and second battery electrode
Construction
Electrodes
Supercapacitor construction is explained on EDLC symmetrical structure. Nevertheless, the basic design concept is also valid for pseudocapacitors that target boosting electrochemical storage using different materials, processes, and electrolytes.
When we apply a voltage over the capacitor, existing ions in the electrolyte go through the membrane to their respective electrode, i.e., to the surface of the activated carbon that, via the electrolyte, is connected to the current supply electrodes. The ions are captured on the activated carbon surface, which attracts reverse charges inside the carbon (Figures 5. and 6.). We thus have a double layer of charges. Hence the name double layer capacitor. A schematic taken from a modern construction is shown in Figure 6. The original designs from the 1970s used a membrane and conductive rubber as a current collector, as shown in Figure 5. Modern constructions replace the membrane with a separating porous foil and the conductive rubber with a current collector. Usually an aluminum foil – see Figure 6. The structure of the wound – the stacked type using aluminum foil as a current collector, is shown in Figure 7.
Figure 5. illustrates how the activated carbon and the ions in the electrolyte work together.
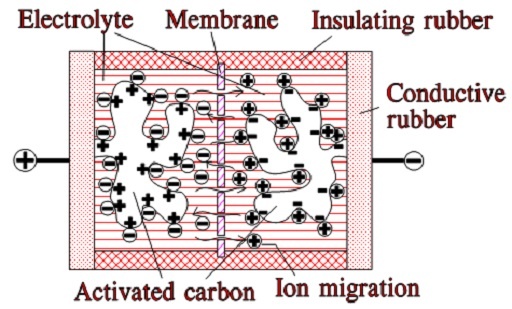
Figure 5. Function of EDLC supercapacitor (older construction)
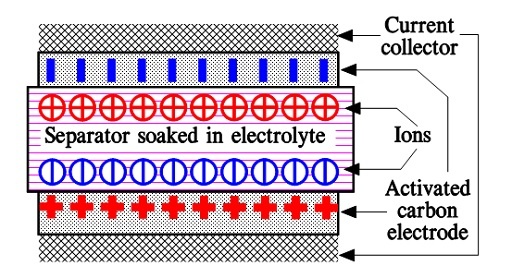
Figure 6. Schematic of EDLC supercapacitor

Figure 7. Wound EDLC supercapacitor structure with aluminum foil current collector
Because the distance between the charges is small – ion diameters –and furthermore, because the total carbon surface is enormous, the charge quantity will be extremely large. The capacitance range amounts to the magnitude of several thousand farads.
The continuous development to enlarge surface area has resulted in sophisticated, active electrode systems based on active carbon layer (carbon fibers), carbon nano-tubes (CNT), or the latest design with graphene.
Electrolyte
Applied voltage, efficiency, and power handling also depend on a selection of electrolytes. Electrolytes provide a media that supports the creation of charge on the interface with electrodes, enables its mobility, or provides adsorbed ions as charge carriers (pseudocapacitance). Electrolyte matching with the electrode system is thus essential to achieve maximum energy and power density and define cell voltage.
There are currently three types of electrolytes:
- aqueous based
- organic-based (liquid or solid/gel)
- ionic liquids
Aqueous electrolytes provide good conductivity at no toxicity. However, the maximum voltage reaches 1.2 V.
Organic electrolytes can have a maximum of ~3 V and provide a better temperature range. Nevertheless, they can be limited by flammability or toxicity. Solid organic electrolytes usually contain conductive polymers with low ESR values and corresponding power pulse capabilities.
The latest electrolyte development steps. Ionic liquids are salts in liquid form rich in ions and short-lived ion pairs. This electrolyte increased maximum voltage to ~3.7 V at no issue with flammability or toxicity – see spider chart benchmarking electrolyte types in Figure 8.
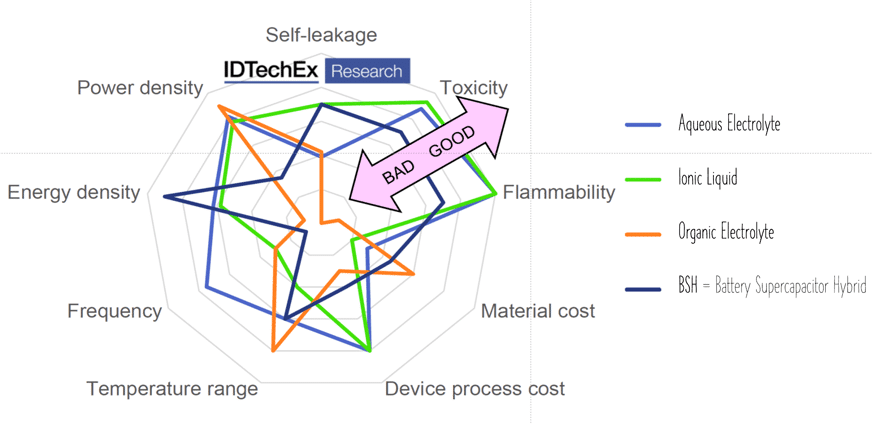
Figure 8. Supercapacitor electrolyte types comparison; source: IDTechEx, used under permission
If the voltage exceeds the maximum cell voltage, the electrolyte decomposes to form H2 and O2. The surge voltage is specified below that voltage, and with some margins to the surge voltage, we find the rated cell voltage.
ESR Resistance
The active electrode (carbon) particles in the dispersion variant connect via the electrolyte and adjacent particles with the current supply electrodes. Some particles are situated close to its electrode and have a comparatively small contact resistance; others have long contact chains and manifold larger connection resistances. Figure 9 shows three particles with the series resistances R1, R2, and Rx, where R1 < R2 << Rx. The figure also shows the membrane resistance Rm in the electrolyte. The carbon particles are not spheres but have a surface with hollows and channels, just like the etched surface of aluminum foil, but even more, enlarged. See the schematic enlargement in the figure.
The electrolyte resistance from the inlet to the bottom of a channel will be considerable. The charges in the channel get a varying contribution of series resistances, depending on location in the channel, a contribution that shall be added to those resistances R1, R2…., intimated in the entire view.
This results in many elementary capacitances mutually connected in parallel in a complicated resistor network whose part resistances differ between themselves with several powers of ten. The time constants of the respective elementary capacitances vary from fractions of a second to hundreds of hours. The resistance network can be summarized as an ESR that varies with capacitance, type, and manufacture from milliohms to several hundred ohms at RT. What contributes to the lower ESR values of modern ultracapacitors is the more conductive organic electrolytes or ionic liquids and improvements in the contact medium between the active electrode particles and current collectors.
Because the ESR in many backup capacitors is large compared to aluminum electrolytic, it limits the ripple current use. The usual limit for the heat release is set to +2 °C.
We don’t have any real dielectric, only a face boundary between electrode and electrolyte of 2 to 5 nm that prevents separate charges from passing.
Series connection
Energy increase with voltage squared; thus, modern high-power electronics require work in 16, 25, 35, 50, and 110-volt ranges, which requires multiple cells linking (2 to 4 V). The automotive market push towards 48-volt subsystems.
That raises concern about the reliability of units containing multiple cells linked together. If we in electronic designs, want to connect discrete capacitors in series to meet higher working voltages, we should use the same type of elements.
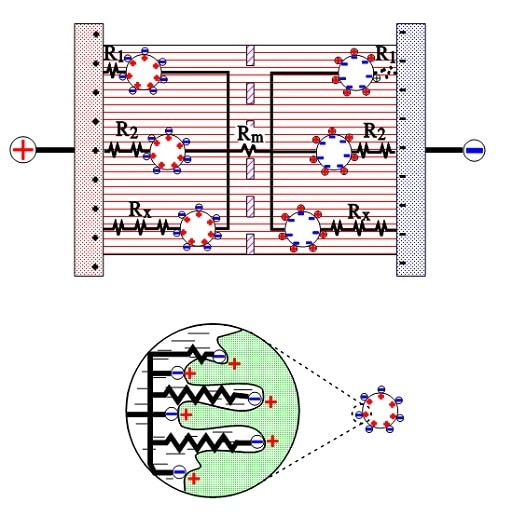
Figure 9. Electrolyte and contact resistances in the double-layer supercapacitor.
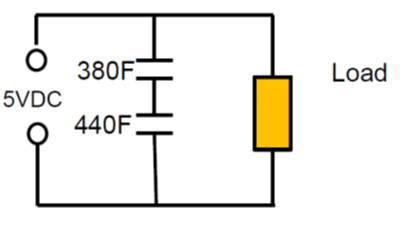
Figure 10. supercapacitor series connection
As an example: Series connection of 2pcs 400F 2.5V cells with +10/-5% cap tolerance in worst case scenario will end up with 380F and 440F caps on the board. The voltage of the individual cells will split accordingly to: 2.68 V: 2.32V, which exceeds the rated voltage of the first capacitor.
As noted from electrolyte decomposition, the rated cell voltage of supercapacitors (or surge voltage, if specified) must not be exceeded. Exceeding the maximum cell voltage considerably lower the lifetime of supercapacitors; thus use of balancing circuits is strongly recommended when connecting unit cells in series to attain higher-rated voltages.
Picked from a manufacturer datasheet: The rule of thumb by EDLC supercapacitor lifetime prediction is:
- With every 0.2 voltage decrease, the cell lifetime increases about 2x in the specified voltage range
- With every 0.1 voltage increase over the spec V, the cell lifetime gets half
see figure11. Comparison of balancing methods and their impact on lifetime and efficiency.
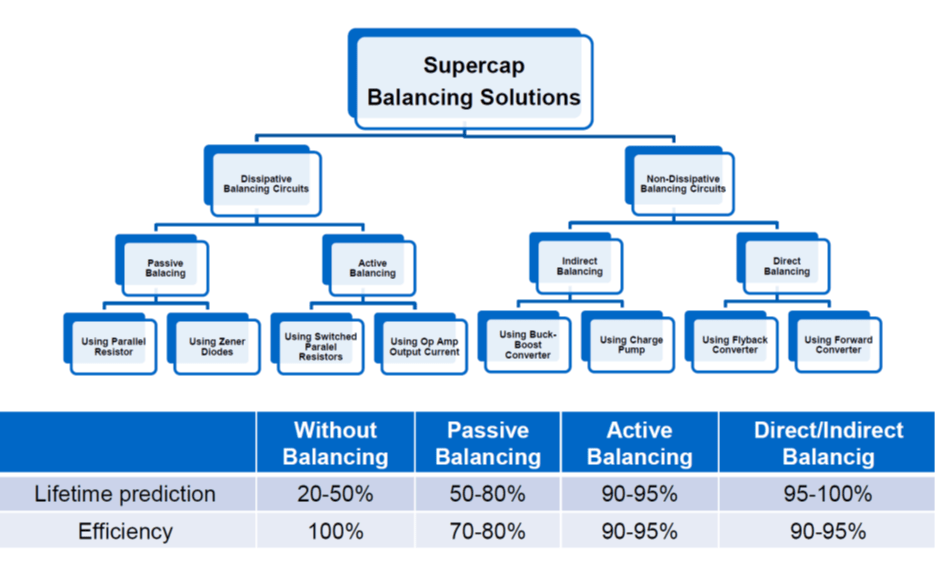
Figure 11. balancing method impact to lifetime and efficiency of EDLC supercapacitors; Source: Eaton
If we don’t use external voltage dividers, it is recommended as a precaution that the applied total voltage divided by the number of linked cells does not exceed 85% of the rated cell voltage.
Note: Low voltage (~1.8V) aqueous electrolyte type may not require balancing on higher voltage modules as the variability of applied voltage on the high number of cells/layer may not be critical.
Source: Epci Blog
